Goals
| Demonstration 1: Disappearing Smoke Items | Amount |
|---|---|
| Giant Jar | 1 |
| Matches | several |
| Incense (or something to make smoke) | optional |
| Isopropyl Alcohol | 2 capfuls |
| Safety Glasses and Gloves | # of students |
| Demonstration 2: Bouncy Ball Items | Amount |
| Borax | 1 teaspoon |
| White Elmer's Glue | clear school glue makes clear ball |
| Warm Water | 6 teaspoons |
| Small plastic mixing cups | 2 |
| Spoon to stir | 1 |
| Measuring Spoons (or just plastic spoons) | 1-2 |
| Zip-lock bag | 1 |
| Cornstarch (optional) | 1 tablespoon |
| Food Coloring (optional) | drop |
There are some topics you may want to go over with students before this lesson.
PLEASE USE GLOVES AND SAFETY GOGGLES WHEN DOING THIS DEMO!!
PLEASE USE GLOVES AND SAFETY GOGGLES WHEN DOING THIS DEMO!!
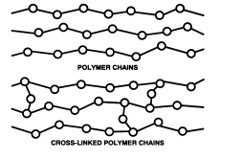
Disappearing Smoke Discussion:
This was an example of a chemical change because of the combustion. This is also an example of a physical change because of the isopropyl alcohol. The alcohol had to vaporize (the way water vapor forms when water boils--kind of like the steam) and we helped speed up this process by shaking the jar.
What characteristics of a chemical reaction did it have?
Concept check question: So to review, what type of reactions did this demo exhibit? Are there any questions?
Bouncy Ball Discussion:
1. Was this a chemical reaction or a physical reaction?
Chemical reaction because of polymer cross linking. Molecules don't normally come together, but because of borax they cross link together.
2. Give an example of each of a chemical reaction and of a physical reaction in everyday life. Explain why they are an example of that type of reaction.
C) Pepsi Mentos (physical reaction) - will have to be done outdoors (10 minutes) Materials:
a) Liter bottle of Diet Coke b) Mentos
1) Place Diet Coke in an open area
2) Drop mentos into Diet Pepsi, and watch it fountain out
Run demo about 5 times:
Do it once with 2-3 mentos. Discuss. Type of reaction? Physical. Why? Was anything produced? Was anything consumed? Nucleation site. Kind of like pushing the first domino in a stack of dominoes. Without that first one none of them will fall.
Do three more times. Let students pick a number and predict what will happen. Demo.
Fifth bottle, predict what will happen, then drop mentos in bottle and close cap.